More than 30 possible coronavirus vaccines are in clinical trials worldwide in a bid to squeeze traditional timelines and roll out a vaccination programme in the next six to twelve months. But there are calls to speed up the process further through the use of controversial human challenge trials where volunteers are given an experimental vaccine, then deliberately exposed to the virus.
Despite real safety risks and ethical concerns, there is a burgeoning appetite for these trials. More than 36,000 people from more than 160 countries have expressed a willingness to take part with 1 Day Sooner, an organisation that supports COVID-19 challenge trials.
The organisation published an open letter in July, urging preparation of such trials, which was signed by more than 150 academics and experts, including 15 Nobel laureates.
But the search for a vaccine suffered a temporary setback in early-September when an Oxford University trial with British drugmaker AstraZeneca was paused after a volunteer suffered a possible serious adverse reaction, illustrating the risks involved even without deliberate exposure to COVID-19.
Abie Rohrig, director of communications at 1 Day Sooner and challenge trial volunteer, says his desire to take part is for the good of humankind. “Since I am at a very low risk of death and hospitalisation from COVID-19, I am more than willing to take on the risk so that older and immunocompromised populations can stay safe,” the 20 year old says.
Human challenge trials could save lives or take them
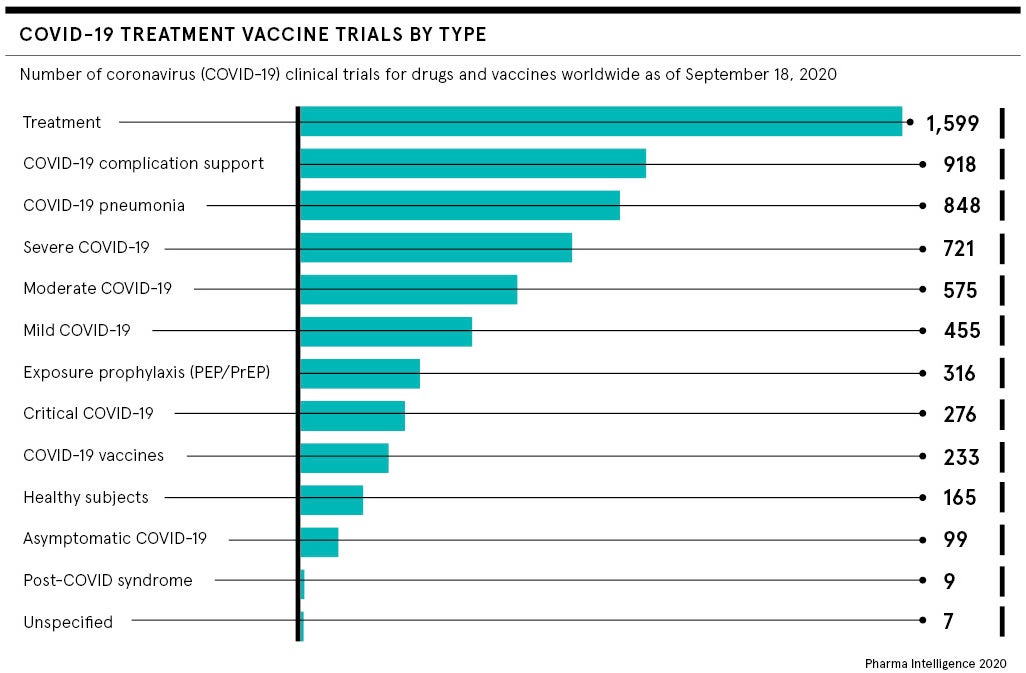
Challenge trials have been used in the past to test vaccines against flu, typhoid, cholera and malaria. They can speed vaccine development when low levels of virus circulating in the population make determining normal vaccine efficacy difficult. They can also compare the efficacy of different vaccines, highlighting which candidates should be advanced, as well as providing important insights into the virus, disease progression and immunity.
A coronavirus vaccine would benefit from all this, particularly if challenged in the low-risk group of healthy 18 to 30 year olds, where fatal infection rates are estimated to be 0.03 per cent. But, unlike malaria and typhoid, COVID-19 has no sufficient rescue treatment should trial volunteers get sick and the SARS-CoV-2 virus is still poorly understood.
Since I am at a very low risk of death and hospitalisation from COVID-19, I am more than willing to take on the risk
The first peer-reviewed paper calling for coronavirus challenge trials was published online in March in The Journal of Infectious Diseases. Professor Nir Eyal, a senior bioethicist and director of Rutgers University’s Center for Population-Level Bioethics in the United States, was the lead author and now a member of the 1 Day Sooner advisory board.
He says there are risks to volunteers, but challenge trials should be supported. “We don’t need challenge trials to beat COVID. We need them to beat it faster,” he says. “Faster means averting thousands of deaths per day.”
The risk too high and the reward uncertain
But the World Health Organization remains undecided and other academics claim now is not the time. Dr Gregory Poland, director of the Vaccine Research Group at the US-based Mayo Clinic, believes the claims about saving lives are false arguments. Even if a challenge trial was conducted today, he says, people would not be protected with a coronavirus vaccine tomorrow. It could take months just to find the appropriate strain and dose for these trials, erasing the speed advantage.
There is also no guarantee the results of challenge trials in low-risk healthy young adults would be applicable to the elderly or those with underlying conditions. “To me, it’s unethical to challenge somebody and we’re in the middle of multiple, large phase-three vaccine trials, so I do not see the benefit compared with the risk,” says Poland.
Dr Michael Jarvis, associate professor in immunology and virology at the University of Plymouth, takes a similar stance. “A bad experience at the early stages of the vaccine development pathway could really impact acceptance of an efficacious COVID vaccine by the public,” he says. “I think this is a legitimate reason to wait.”
Supporting challenge trials can be controversial
Yet we are getting closer to COVID-19 challenge trials. Both US and European regulators say they will consider proposals, and pharmaceutical company Johnson & Johnson says it is “evaluating the potential benefit of human challenge trials”. The US National Institute of Health also recently announced the decision to explore the possibility of, and prepare for, human challenge trials.
Meanwhile, the Oxford University group developing the frontrunner coronavirus vaccine is understood to be reviewing the technical aspects of how these studies could be undertaken.
A cross-national survey of almost 6,000 people conducted by Eyal and colleagues in July found 75 per cent preferred scientists to conduct challenge trials over standard trials, even as respondents acknowledged the risks. The results may help remove a roadblock to their implementation. It could also open the door for more widespread use in infectious diseases and future pandemics, especially in the absence of rescue treatments.
The problem though, says Eyal, is people are cagey about their support. “Nobody wants to be first, lest the public and electorates punish them for it,” he says.
Can we afford to wait longer for a vaccine?
But we may have no choice. Officials in America have already said challenge trials might be needed if natural infections decline and standard studies can’t determine efficacy. Ana Nicholls, healthcare analyst and managing editor of the Industry Briefing at The Economist Intelligence Unit, agrees this is a concern. “Some vaccine developers have had to chase cases around the world to set up and conduct trials. Some early trials were abandoned altogether,” she says.
If we want a vaccine now or for a future pandemic, challenge trials may be the only option, but balancing risk against the potential benefits is tricky. COVID-19 could shift the needle.
1Day Sooner have just submitted a formal UK petition in support of challenge trial preparation which has found support among prominent figures in UK medicine. Lord Ara Darzi, Nobel Laureat, Sir Richard Roberts, and Dominic Wilkinson (the head of medical ethics at Oxford) have all signed.
Rohrig at 1 Day Sooner is optimistic, believing it’s likely a challenge trial will begin by the end of the year. Tens of thousands of young people have acknowledged the risks and still want to take part, he says. “We hope others do not speak on our behalf as to which risks are justifiable for us to take,” Rohrig adds.
How COVID has changed clinical trials
Coronavirus lockdowns forced the world to stay at home. This also caused the pause and postponement of thousands of clinical trials, with patient enrolment in trials significantly down, by as much as 79 per cent in April compared to the previous year, according to Medidata.
But the lockdowns have also spurred innovation in the sector. Traditional timelines, for instance, are being busted in a bid to get a coronavirus vaccine to market through greater collaboration between companies and different trial designs. In the United States, the collaborative project Operation Warp Speed aims to deliver a vaccine by the end of the year by running design, manufacturing and delivery operations in parallel rather than sequentially.
Additionally, clinical trials have moved outside traditional trial sites with a boom in virtual trials, which use remote monitoring, teleconferencing and digital data collection tools, such as wearable devices. Artificial intelligence (AI) is also beginning to be used in the recruitment process to identify patients suitable for trials.
The COVID-19 legacy will be a positive one, says Graham Belgrave, senior vice president, head of European operations, at clinical research organisation Advanced Clinical: “I believe the genie is out of the bottle and we will see an ever-increasing reliance on AI, on remote and virtual visits for clinical trials.” This, he says, is likely to become the new norm.

More than 30 possible coronavirus vaccines are in clinical trials worldwide in a bid to squeeze traditional timelines and roll out a vaccination programme in the next six to twelve months. But there are calls to speed up the process further through the use of controversial human challenge trials where volunteers are given an experimental vaccine, then deliberately exposed to the virus.
Despite real safety risks and ethical concerns, there is a burgeoning appetite for these trials. More than 36,000 people from more than 160 countries have expressed a willingness to take part with 1 Day Sooner, an organisation that supports COVID-19 challenge trials.
The organisation published an open letter in July, urging preparation of such trials, which was signed by more than 150 academics and experts, including 15 Nobel laureates.
