The first thing to be said about rare diseases is that they are not actually that rare. A rare disease is defined by the European Union as one that affects fewer than five in ten thousand of the general population. That may sound like your chances of being directly affected are very small indeed. But there are thousands of known rare diseases and more are being designated each week.
In fact, more than 3.5 million people in the UK will be affected by a rare disease at some point in their lives, which is comparable to the number of people with diabetes.
Yet while awareness of diabetes is high and the NHS has clear care pathways for that condition, this is certainly not true of rare diseases. All too often a diagnosis is just the beginning of a battle for access to what are known as orphan drugs for rare diseases, which can add many years to life expectancy as well as improve quality of life.
This is problematic, for the aim of clinical research in the 21st century is, effectively, to turn conditions such as cancer into rare diseases.
The high cost of personalised medicine
Personalised medicine, which is seen as the future of healthcare, is about tailored treatment for individual patients based on their genetic signatures and clinical characteristics. This means breaking down a condition such as breast cancer into hundreds or thousands of genetic mutations and finding an effective treatment for each one, just as we do for rare diseases at present.
Patients do better and the NHS wastes less money on drugs and medicines that we know do not work.
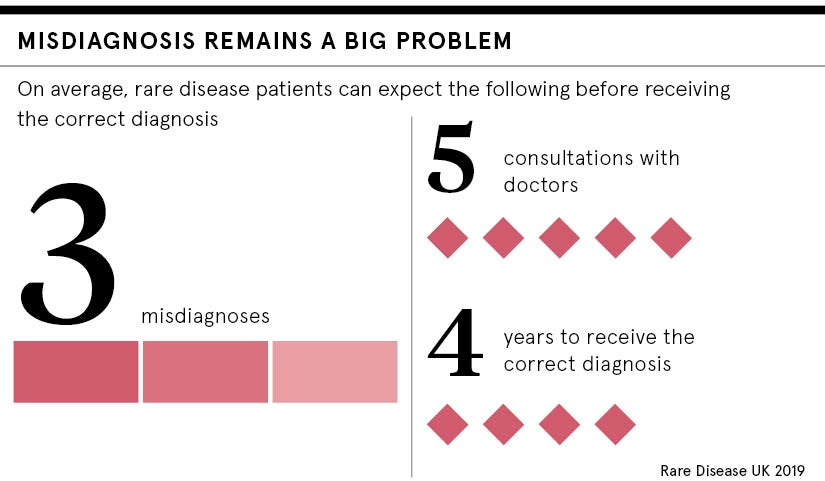
The UK has made progress in recognising the importance of providing care for people with rare diseases in recent years, with an increased focus on early diagnosis, better care and information sharing. But it still lags behind other EU countries in developing the right systems to bring these treatments through to patients.
Take cystic fibrosis, one of the better-known rare diseases. Cystic fibrosis is a genetic disorder that most affects the lungs, but also the pancreas, liver, kidneys and intestine. There is no known cure, however a new type of drug has been developed that makes a profound difference to about 40 per cent of patients, who have a particular genetic mutation.
But Orkambi remains out of reach in the UK, to the frustration of patients and their loved ones. The reason? Price. There is no doubt that Orkambi does what it says on the tin. But at £104,000 a year, for life, the watchdog National Institute for Health and Care Excellence (NICE) has decided that it does not represent value for taxpayers’ money.
Orkambi has been approved in Europe and a number of countries in the EU, including France and Germany, make it available to patients. But in England there is a standoff between the NHS and the manufacturer of Orkambi, Vertex Pharmaceuticals, which claims the cost reflects the investment in research and development of a drug for a small patient population.
Long-term plan for rare diseases medicine
Can these positions ever be reconciled? The answer is of immense importance, not just to cystic fibrosis patients, but to patients suffering from what we now consider to be rare diseases.
As personalised medicine becomes more widely available, the future of the NHS will depend on equitable access to high-quality services, treatment and support. New cancer drugs which, like Orkambi, target the underlying genetic causes of the disease, will also cost significantly more than earlier treatments, presenting NICE with challenges to its existing approval criteria.
NICE has a Highly Specialised Technology evaluation, which has seen a number of innovative pricing and reimbursement deals done with companies to bring drugs for rare diseases to NHS patients. However, many rare diseases do not qualify for this pathway and must, therefore, be appraised via its standard Single Technology Appraisal (STA) process.
According to market access consultancy MAP BioPharma, just 13 per cent of the 24 completed STA reviews of rare disease medicines between 2013 and 2017 were recommended for the full eligible population. This compares with a full recommendation for more than two thirds of other medicines.
The UK’s strategy for rare diseases has a strong focus on addressing unmet need through the discovery of new drugs. The jewel in the crown is the 100,000 Genomes Project, which is sequencing whole genomes from NHS patients. The project’s focus is on rare diseases, as well as some common cancers and infectious diseases. The full 100,000 milestone was reached in December 2018.
The continuing progress of the project and the concurrent development of a genomic testing strategy will underpin the development of new genomic medicine services for the NHS. This is a long-term investment in the future of healthcare through personalised, targeted treatment, but the knowledge harnessed by the project is already yielding benefits.
NHS’s uphill battle for equitable access to medicine
In June, Simon Stevens, chief executive of NHS England, announced that the health service was preparing to fast track tumour-agnostic drugs that target tumours according to their genetic make-up, rather than where they originate in the body.
Last year NHS England signed a landmark commercial deal to make pioneering CAR-T therapy available to cancer patients. CAR-T is a type of immunotherapy, which involves collecting and using the patient’s own immune cells to treat their condition.
Mr Stephens says: “These exciting new breakthroughs in cancer treatment are the latest example of how the NHS can lead the way in the new era of personalised cancer care.
“Preparations are underway to make sure the NHS can adopt this next generation of treatments, but manufacturers need to set fair and affordable prices so treatments can be made available to those who need them.”
Finding ways to ensure patients with rare diseases have equitable access to the drugs and treatments they need is a significant challenge for the NHS. But it also offers a great opportunity. For helping people stay healthier for longer is key to creating a sustainable health and care service.
The high cost of personalised medicine