The early diagnosis of a rare disease can help people with such conditions to improve their quality of life. When this happens shortly after birth, it can enable life-saving interventions and prevent severe disabilities from developing.
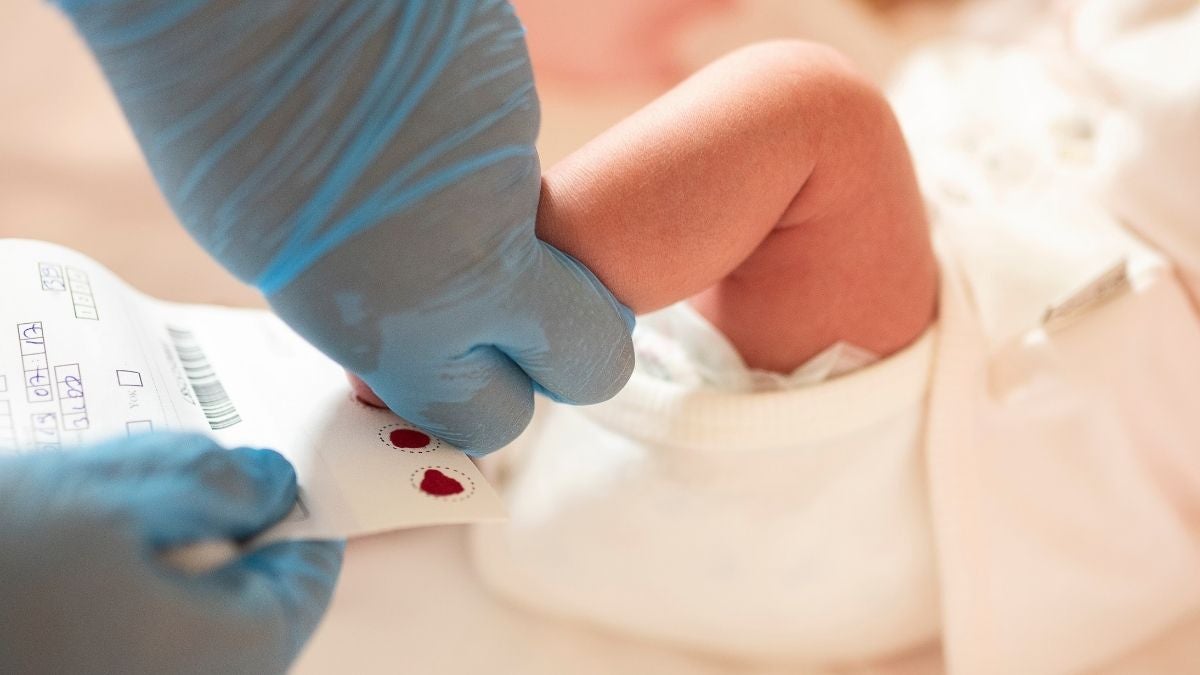
All babies in the UK are offered newborn blood-spot screening on the NHS, also known as the heel-prick test, which is ideally conducted when they’re five days old.
This test typically screens for nine rare but serious conditions: sickle-cell disease, cystic fibrosis, congenital hypothyroidism and six inherited metabolic diseases. (In some parts of England, it also covers severe combined immunodeficiency.) But Professor Jim Bonham, president of the International Society for Neonatal Screening, says that the “genetics revolution” means that 300 to 400 more conditions could be covered.
“I think we should certainly explore the potential that genetics offers, but do so in a way that serves patients’ interests,” he says. “We need clear evidence to demonstrate that, by intervening early in life, we are going to offer real benefits for the child.”
Bonham adds that newborn screening is best applied to treatable diseases where early diagnoses would offer improved outcomes.
“It is very important to maintain public confidence,” he stresses. “Some parents may not wish to know about a condition if no treatment for it is available. Others may wish to know, even if there’s no treatment, to enable them to plan effectively for the future of their family. But all would agree that the early diagnosis of a serious disorder that leads directly to life-changing treatment is to be welcomed. This is the central remit of newborn screening.”
A pilot programme backed by NHS England is set to explore the potential for extending the scope of the heel-prick test. With support from UK Research and Innovation’s Sciencewise programme, the UK National Screening Committee and Genomics England, the body established to deliver the 100,000 Genomes Project, have invited the public to comment on their proposed use of whole-genome sequencing in newborn screening. The findings of the consultation so far indicate widespread support for the move, providing that the right safeguards and resources are in place.
The UK National Screening Committee, which reviews its recommendations on testing for different conditions as new research findings become available, advises the NHS on which screening programmes to offer. When considering whom to test and which conditions to test for, the benefits of offering such a programme are weighed against the potential harms. The committee recommends screening only when it believes that the benefits to those being screened will outweigh the harms.
Some parents may not wish to know about a condition if a treatment is not available
The ability to sequence and analyse a newborn’s entire genetic code could enable clinicians to detect many more conditions and transform the NHS into a more prevention-focused healthcare system. But the proposal raises ethical and societal questions, which is why the committee and Genomics England are seeking the public’s input before considering the use of whole-genome sequencing in the NHS. There will also be extensive engagement with clinicians and patients to shape the programme.
“The difficult questions concern how predictive the results are; which conditions it would be acceptable to look for; what information to give to whom and when; and how to help parents make informed choices about tests that could have important implications for their child, themselves and others,” explains Professor Bob Steele, chair of the UK National Screening Committee.
Professor Sir Mark Caulfield, who recently stepped down from Genomics England after eight years as its chief scientist, adds: “This work is a fantastic foundation from which to take forward the exploration of a pilot newborn programme.”
The next step of this project will be to design and run an ethically approved research pilot embedded in the NHS to explore whether and how to offer whole-genome sequencing to all newborns to accelerate diagnosis and access to treatments for rare genetic conditions. Up to 200,000 babies’ genomes will be sequenced and analysed for a set of actionable conditions that may affect their health in infancy.
With parents’ consent, babies’ genomes will be de-identified and added, alongside their health data, to the National Genomic Research Library that Genomics England manages. This information will help vetted academic, clinical and commercial healthcare researchers to improve their knowledge; develop new tests and treatments; and understand how therapies can be improved or repurposed.
Storing babies’ genomes securely, regardless of their screening outcome, could also enable these to be reanalysed as needed, potentially enabling access to new developments in genomics throughout their lifetimes.
The scale and delivery quality of newborn screening programmes can vary drastically from one country to another. Testing for more than 50 diseases, Italy leads the way in Europe, with Austria, Hungary and Spain testing for 20 or more.
Eurordis, an alliance of nearly 1,000 patient organisations in the UK and 73 other nations, advocates a harmonised approach to newborn screening as the only way to ensure that as many babies as possible are covered by the most comprehensive tests available.
This would “guarantee the human right of achieving the highest standard of health for all newborns, regardless of their country of birth”, says Eurordis board director Simona Bellagambi, who has a teenage niece with tuberous sclerosis, a rare and currently incurable genetic condition. “If you could help your baby by reducing the severity of their disease, or possibly even help them to be healthy, wouldn’t you try your best? Screening is an effective way to obtain a diagnosis – and all newborns deserve it.”

The early diagnosis of a rare disease can help people with such conditions to improve their quality of life. When this happens shortly after birth, it can enable life-saving interventions and prevent severe disabilities from developing.
All babies in the UK are offered newborn blood-spot screening on the NHS, also known as the heel-prick test, which is ideally conducted when they’re five days old.
This test typically screens for nine rare but serious conditions: sickle-cell disease, cystic fibrosis, congenital hypothyroidism and six inherited metabolic diseases. (In some parts of England, it also covers severe combined immunodeficiency.) But Professor Jim Bonham, president of the International Society for Neonatal Screening, says that the “genetics revolution” means that 300 to 400 more conditions could be covered.
