
Big Pharma is having a moment. News of a coronavirus vaccine is casting the pharmaceutical industry in a new light, as collaborative and working in the public interest. It’s a world away from previous perceptions of putting profits before people, keeping secrets and taking time to innovate.
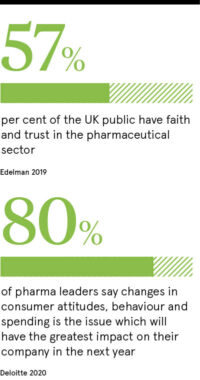
Indeed, the 2019 Edelman Trust Barometer found only 57 per cent of the UK public had faith and trust in the sector. More recently, a survey of UK consumers by medication review site Drugs Disclosed revealed 93 per cent of patients are mistrustful of information about their medication, with 84 per cent believing the pharma industry influences prescription decisions.
What potential, then, does the vaccine have to redeem the pharmaceutical industry’s reputation and how can this reinvigorated trust unleash its true potential? More importantly, how can Big Pharma maintain this trust post-Covid?
Continuing momentum to maintain positive perception
Consensus is that the collaboration, openness and public benefit demonstrated in creating a vaccine are what stands to rehabilitate the industry’s reputation, with more of this needed beyond its rollout.
“The events of 2020 have given the public a window into the world of pharma research and development and, for many, have enhanced their view of the sector,” says Dr Steve Arlington, president of pharmaceutical collaboration network the Pistoia Alliance.
“The importance of setting up collaborative programmes between companies that would normally compete was quickly recognised by the industry. The recent announcement by Pfizer is one of, we hope, many that will be made over the next few months and this success proves collaboration is hugely important in our quest to cure, treat and prevent disease.”
The sector must carry this momentum and collaborative instinct forward to continue building trust, he adds.
This attitude needs to extend to the general public, notes Professor Sam Shah, chief medical strategy officer of men’s health specialists Numan, with deeper transparency required on a range of levels.
“Advances in healthcare are likely to come from real-world evidence and citizen data. However, for citizens to share their data they need to know how it’s going to be used, for what benefit and what outcome. Increased transparency within the sector would build this trust, but requires collaboration between regulators, industry and the public,” says Shah.
“Mechanisms could include platforms where citizens control their own data, with visibility of who accesses it. These sorts of platforms don’t yet exist in the UK or most parts of the world.”
The incentive model for pharmaceutical companies must also move towards better patient outcomes and community investment, versus the current transactional model, allowing for better alignment with the public system, he adds.
A shift in the industry has to start with leadership at the top; they have to become more human
“There needs to be a better way of matching needs to the pipeline. However, this requires a new relationship of co-production, one many public healthcare systems struggle with. They generally have a poor record of managing commercial relationships or working with industry,” says Shah. It boils down to better engagement with citizens and the healthcare community overall.
Karina Malhotra, founder and managing director of patient experience consultancy Acumentice, believes this is easier said than done.
“Renewing an image that doesn’t put profits against the greater good is key. It’s the communication of that social-value piece, without coming across as opportunistic, that remains elusive,” she warns.
Breaking the mould to transform the industry’s reputation
A handful of pharmaceutical corporations are breaking the mould. Chiesi, for example, has become the largest pharma company to obtain B Corp accreditation. And, as Sana Alajmovic, founder and chief executive at preventative healthcare startup Sigrid Therapeutics, points out, they are already gaining ground in boosting the pharmaceutical industry’s reputation.
“Merck’s CEO [Ken Frazier] was part of a business advisory council in the Trump administration and when the President refused to crack down on white supremacist violence that was happening in 2017, he simply stepped away from that council,” Alajmovic recalls.
“And Novartis’ CEO [Vasant Narasimhan] is a great example of a modern leader. He has his own Instagram account, where he communicates with staff, shares books he has read; it’s like he’s talking to you. “So a shift in the industry has to start with leadership at the top; they have to become more human. They have to take a stand because you don’t operate in a vacuum. There are a lot of forces at play, like sustainability and leadership, through which pharma will have to change the way it works, to be better perceived.”
B Corps and Big Pharma

Becoming a B Corp is no easy feat, yet those who do can claim the highest standards of social and environmental performance, public transparency and legal accountability to balance profit and purpose.
With more than 3,500 companies worldwide now certified as B Corps, just 20 are in pharmaceuticals. The largest is Italian conglomerate Chiesi. Group president Alberto Chiesi believes coronavirus has accelerated a cultural shift from “shareholder capitalism” to “stakeholder capitalism”, and Chiesi has a role to play to attract more companies to “embrace this way of doing and measuring business performance”.
“The trigger point is when it will be clearly acknowledged that striving to generate shared value does not mean businesses should focus on positive impact at the expense of financial performance. Acting as a sustainable business is a key component of a long-term success strategy. That deserves also a concrete reflection on how forward-looking policies could support and reward sustainable business models,” he says.
Yet Dr Steve Arlington, president of the Pistoia Alliance, caveats that becoming a B Corp is just one element of repairing the pharmaceutical industry’s reputation. Co-ordinated efforts both across and outside the sector are crucial for lasting change.
“Companies need to be able to work closely and non-competitively with groups including other pharmas, regulators, patient advocacy groups, health payers and providers, and logistics and supply,” he says.
“It’s important to look outside of the sector, too. The crossover between technology and research and development, in areas like artificial intelligence and machine-learning, telemedicine and quantum computing, is growing all the time. We can only make sure everyone benefits from these innovations if we work together to put in place frameworks to guide adoption and pool resources.”
