
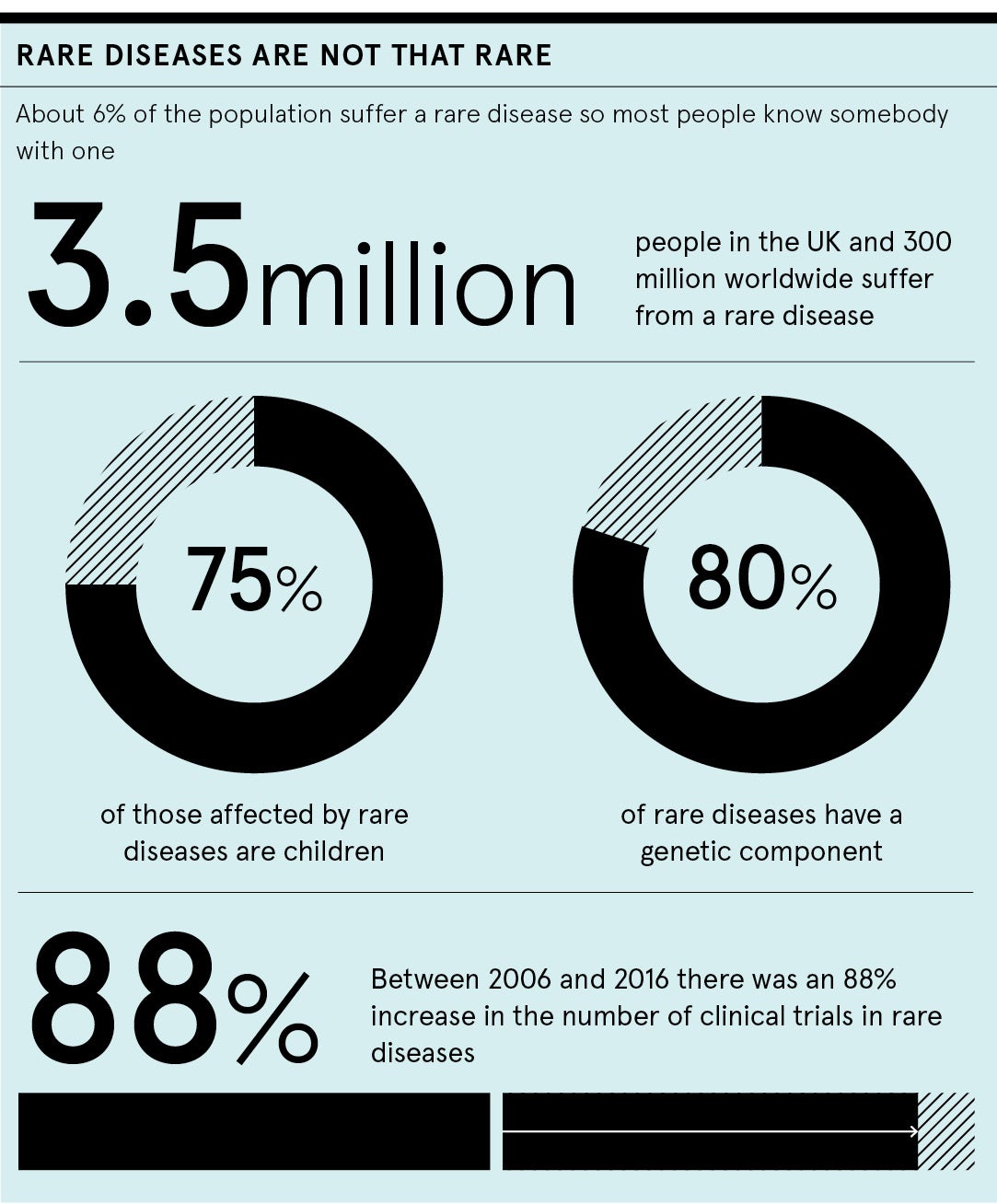
The potential of new and emerging treatments in the rare disease field offers real hope for the 3.5 million people in the UK and 300 million people worldwide who will be affected by a rare disease at some point in their lives. It’s a positive sign that between 2006 and 2016 there was an 88% increase in the number of clinical trials in rare diseases. But innovation in clinical trial operations needs to be prioritised, so that the study of rare diseases in human subjects is simpler and more modern, paving the way to get potential treatments to patients faster. For those living with rare diseases, it’s highly frustrating that drug development is such a slow process. It can take years to develop new treatments, which means efficiency of clinical trials for rare diseases is essential.
Despite their collective name, rare diseases are, paradoxically, quite common overall. About 6% of the population suffer a rare disease, so most people know somebody with one. Of the 7,000 or so rare diseases that we are currently aware of, some are well known due to the raised media profile they have. For example, cystic fibrosis and motor neurone disease, have more notoriety than other rare diseases thanks to actors such as Jenny Agutter who has the cystic fibrosis gene, and sportspeople such as ex-rugby player Doddie Weir and ex-footballer Stephen Darby who both have motor neurone disease. Sadly, 75% of those affected by rare diseases are children. For example, Duchenne muscular dystrophy is a horrible, life-limiting disease of boys that often starts very early in childhood.
It is essential for physicians to have immediate access to determine whether the person in front of them meets the eligibility criteria of a rare disease clinical study
One of the challenges of any clinical trial – especially for rare diseases – is the recruitment phase. This is when researchers must find enough people who match the characteristics to be eligible to participate in the study. This means it is essential for physicians to have immediate access to determine whether the person in front of them meets the eligibility criteria of a rare disease clinical study. The fact that study documents are stored on paper away from the clinic is not conducive to the real-time decision making necessary to enrol candidates in complex studies.
We should also consider that 80% of rare diseases have a genetic component. Hope now rests on some of the new advanced therapies, such as gene therapy and cell therapy. We already have a gene therapy for a rare form of blindness helping to transform people’s lives. In addition, we are moving closer towards being able to alter genes in people with genetic rare diseases through CRISPR-Cas9 gene editing. This involves changing a single letter, or base, in a gene. Furthermore, CAR T-cell therapies which use gene-replaced autologous T-cells, have been approved in the last couple of years as part of treatment for some rare types of cancer.
These approaches offer real promise for future progress in rare disease treatment. But modern medicine also requires more sophistication in clinical trials. A digital, mobile-first approach to clinical trials equips investigators and other research staff with the information they need to make informed decisions and properly carry out complex procedures. This makes real-time communication with experts even more critical to preserve the data integrity of the clinical trial and more importantly protect the safety of the human beings in the study.
A person living with a rare disease has a basic human right to not have their condition overlooked, no matter how rare. It’s important that such patients are not marginalised and that we respect those with rare diseases. Clinical trials, especially for rare diseases, need to be done in a modern way that avails of the mobile digital tools that we all use in our everyday lives. Otherwise, they will continue to be slow and delay effective treatments.
Teckro clinical trial software provides a hub of communication and collaboration for clinical trial study teams and researchers, ensuring accurate, current study guidance is always available at the point of care.
For more information visit teckro.com
Promoted by Teckro

The potential of new and emerging treatments in the rare disease field offers real hope for the 3.5 million people in the UK and 300 million people worldwide who will be affected by a rare disease at some point in their lives. It’s a positive sign that between 2006 and 2016 there was an 88% increase in the number of clinical trials in rare diseases. But innovation in clinical trial operations needs to be prioritised, so that the study of rare diseases in human subjects is simpler and more modern, paving the way to get potential treatments to patients faster. For those living with rare diseases, it’s highly frustrating that drug development is such a slow process. It can take years to develop new treatments, which means efficiency of clinical trials for rare diseases is essential.
Despite their collective name, rare diseases are, paradoxically, quite common overall. About 6% of the population suffer a rare disease, so most people know somebody with one. Of the 7,000 or so rare diseases that we are currently aware of, some are well known due to the raised media profile they have. For example, cystic fibrosis and motor neurone disease, have more notoriety than other rare diseases thanks to actors such as Jenny Agutter who has the cystic fibrosis gene, and sportspeople such as ex-rugby player Doddie Weir and ex-footballer Stephen Darby who both have motor neurone disease. Sadly, 75% of those affected by rare diseases are children. For example, Duchenne muscular dystrophy is a horrible, life-limiting disease of boys that often starts very early in childhood.
It is essential for physicians to have immediate access to determine whether the person in front of them meets the eligibility criteria of a rare disease clinical study

